"Yolks" and "shells" improve rechargeable batteries
Aluminium could give a big boost to capacity and power of lithium-ion batteries
One big problem faced by electrodes in rechargeable batteries, as they go through repeated cycles of charging and discharging, is that they must expand and shrink during each cycle — sometimes doubling in volume, and then shrinking back. This can lead to repeated shedding and reformation of its “skin” layer that consumes lithium irreversibly, degrading the battery’s performance over time.
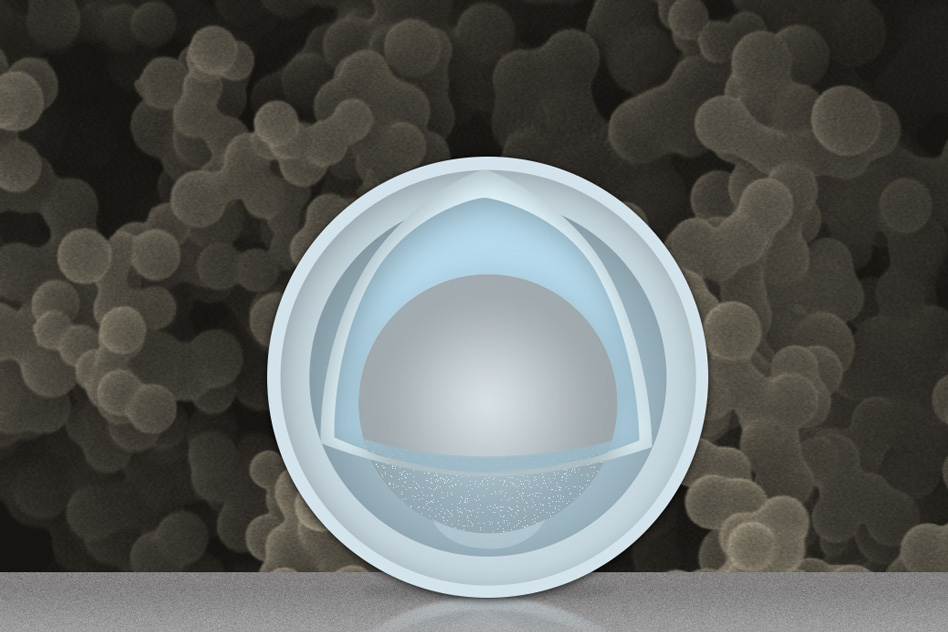
The new findings, which use aluminium as the key material for the lithium-ion battery’s negative electrode, or anode. The use of nanoparticles with an aluminium yolk and a titanium dioxide shell has proven to be “the high-rate champion among high-capacity anodes,” has been reported by the researchers.
The grey sphere at the center represents an aluminium nanoparticle, forming the "yolk." The outer light-blue layer represents a solid shell of titanium dioxide, and the space in between the yolk and shell allows the yolk to expand and contract without damaging the shell. In the background is an actual scanning electron microscope image of a collection of these yolk-shell nanoparticles.
For more details: http://materialsphysics.alliedacademies.com/
One big problem faced by electrodes in rechargeable batteries, as they go through repeated cycles of charging and discharging, is that they must expand and shrink during each cycle — sometimes doubling in volume, and then shrinking back. This can lead to repeated shedding and reformation of its “skin” layer that consumes lithium irreversibly, degrading the battery’s performance over time.

The new findings, which use aluminium as the key material for the lithium-ion battery’s negative electrode, or anode. The use of nanoparticles with an aluminium yolk and a titanium dioxide shell has proven to be “the high-rate champion among high-capacity anodes,” has been reported by the researchers.
The grey sphere at the center represents an aluminium nanoparticle, forming the "yolk." The outer light-blue layer represents a solid shell of titanium dioxide, and the space in between the yolk and shell allows the yolk to expand and contract without damaging the shell. In the background is an actual scanning electron microscope image of a collection of these yolk-shell nanoparticles.
For more details: http://materialsphysics.alliedacademies.com/
Comments
Post a Comment