Tet2 Induces Bone Cell Differentiation by Interacting with Runx1 and Regulates Genomic 5-Hydroxymethylcytosine (5hmC)
Ten-Eleven Translocation two (TET2) is one amongst the foremost often mutated genes in adult myeloid malignancies, together with myelodysplastic syndrome (MDS), myeloproliferative tumor (MPN), chronic myelomonocytic leukaemia (CMML) , and acute chronic leukemia (AML) . TET2 is additionally found to be mutated in T–cell cancer (such as angioimmunoblastic T cancers) and B-cell non-Hodgkin lymphomas (such as diffuse massive B-cell lymphoma and mantle cell lymphoma) .
Notably, TET2 mutations are rife in healthy aged people aged >70 years (∼5%) and are related to clonal haematopoiesis . Tet2 deficiency in mice leads to increased haematogenic somatic cell (HSC) self-renewal. what is more, Tet2 loss skews the differentiation of haematogenic stem/progenitor cells (HSPCs) towards granulocytic/monocytic lineage, often leading to monocytosis and accumulation of monocytes/macrophages in bone marrow (BM) and spleen of mice . Fusion of the monocyte-macrophages result in the formation of multinucleated osteoclasts, the first bone-resorbing cells in mammals. Therefore, it'd be inherently necessary to look at the impact of Tet2 loss on osteoclast genesis and bone mass.
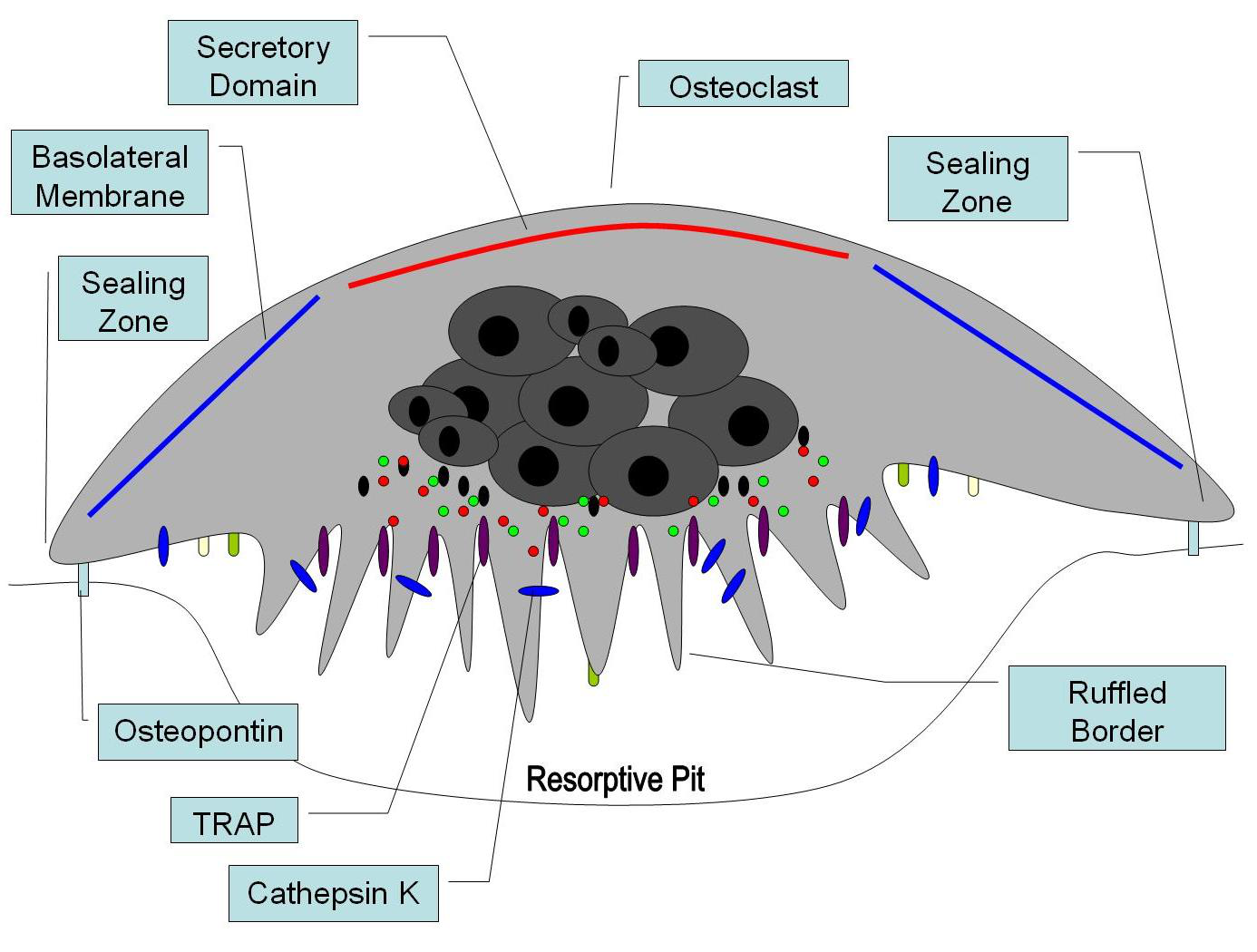 Figure 1: Bone Cell Differentiation
Figure 1: Bone Cell Differentiation
DNA methylation mediated by Dnmt3a, a de novo methyltransferase, plays a task in osteoclasto genesis [16]. Tet2 catalyses the conversion of 5-methylcytosine (5mC) to 5-hydroxymethylcytosine (5hmC) , and conceivably, Tet2 loss or loss-of-function mutations end in aberrant 5mC and 5hmC identification of deoxyribonucleic acid, raising the likelihood that Tet2 could also be concerned within the epigenetic regulation of osteoclast genesis. during this study, we investigated the role of Tet2 in osteoclasto genesis exploitation antecedently established Tet2 knock-out (KO) mouse models. Our findings demonstrate that Tet2-deficient monocyte-macrophages do not differentiate expeditiously into mature bone-resorbing osteoclasts.
Additionally, Tet2−/−mice exhibit enhanced bone mass, probably due to fewer osteoclasts present in vivo. RNA-seq analysis and genome-wide identification of 5hmC on wild type (WT) and Tet2−/−macrophages reveal that Tet2 loss results in important alterations in gene expression (such as Cebpa, Nfkbiz, Mafb, and Id2) and 5hmC identification, with specific enrichment for genes regarding bone cell differentiation. furthermore, Tet2 physically interacts with Runx1, and negatively modulates its transcriptional activity. This study reveals the vital role of Tet2 in bone cell differentiation and performance, implicating Tet2 within the regulation of bone transforming. Thus, Tet2 is expected to be a possible therapeutic target in bone metabolic disorders with altered bone cell activity love congenital disease and osteoporosis.
Comments
Post a Comment